Abstract
BACKGROUND: A widely recognized culprit in chronic lymphocytic leukemia (CLL) pathogenesis is the tumor microenvironment (TME), where an active crosstalk between B-CLL lymphocytes and surrounding cells promote activation of pro-inflammatory master regulators. Recently, our group has described the aberrant expression of S100A9 in B-CLL cells, which correlates with poor therapeutic outcome and NF-kB activation. S100A9 is a damage-associated molecular pattern, and under physiological conditions this protein is constitutively expressed in myeloid cells. Several studies suggest that S100A9 is a potent mediator of inflammation, tumor invasion, and metastasis. Myeloid derived suppressor cells (MDSC) express elevated levels of S100A9, and this is associated with heightened suppressive capacity. Furthermore, S100A9-/- murine model shows higher T cell cytotoxic activity and less accumulation of MDSC. Several studies have also demonstrated that S100A9 promotes a permissive TME through upregulation of programmed death ligand-1 (PD-L1). We hypothesized that expression of S100A9 in B-CLL cells render a survival advantage through activation of inflammatory pathways and modulation of the TME.
METHODS: Eu-TCL1 and S100A9-/- mice were crossed to generate a new Eu-TCL1/S100A9-/- murine model. Adoptive transfer (AT) into NOD-scid IL2Rgammanul (NSG) mice were performed using 5x10^6 of isolated CD19+/CD5+ cells from aged Eu-TCL1 and Eu-TCL1/S100A9-/-. For paquinimod (PaQ) treatment we transferred 15x10^6 Eu-TCL1 splenocytes into C57BL/6 mice. PaQ was administered at a dosage of 25 mg/Kg in drinking water ad libitum for 4 weeks. Once the treatment was completed the animals were euthanized for tissue collection. Fluorescence-activated cell sorting (BD FACS Aria II) were used to isolate CD19+ cells from the spleen of PaQ treated and control mice for Nanostring study. All immunophenotyping analysis was performed via flow cytometry, using a BD FACSymphony or a BD LSR II cytometer.
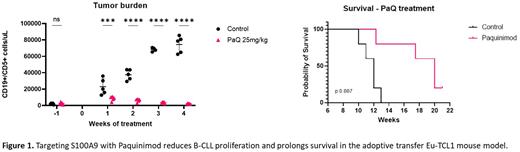
RESULTS: First, we evaluated the expression of S100A9 in normal B cells (CD19+CD5-) and leukemic CD19+CD5+ from Eu-TCL1 mice. We found that similar to human B-CLL cells, the leukemic lymphocytes from Eu-TCL1 mice express high levels of S100A9 protein. Then, we AT CD19+/CD5+ cells from Eu-TCL1 or Eu-TCL1/S100A9-/- mice into NSG mice. Our results show that murine S100A9-/- B-CLL cells grow significantly slower than B-CLL cells with a wildtype S100A9 protein. Moreover, NSG mice from the Eu-TCL1/S100A9-/- group show longer survival compared to the Eu-TCL1 control. Next, we evaluated the in vivo response to the S100A9 inhibitor paquinimod. Our results show a significant lower tumor burden in PB (Fig. 1), spleen, and BM in the mice treated with PaQ compared to the vehicle group. Additionally, longer survival was achieved in the PaQ treated mice. Median survival (MS) was 12 weeks in the control group, while in the PaQ the MS was 20 weeks (Fig. 1). Interestingly, PaQ reduced expression of PD-L1 and impaired production of the regulatory cytokine IL-10 by B-CLL cells. We also observed a reduction in the total number of myeloid cells due to a decrease mainly in the patrolling monocyte subpopulation. Furthermore, we evaluated whether the T cells were affected by PaQ treatment using t-distributed stochastic neighbor embedding (t-SNE) analysis. Seventeen clusters were identified in total. Terminally differentiated CD4+ and CD8+ T cells were the main two subsets found in the control group, whereas naïve CD4+ and CD8+ T cells were predominately present in the PaQ treated mice. Likewise, less accumulation of Tregs and lower expression of exhaustion markers (Eomes, KLRG-1 and TOX) were observed in the cohort that received PaQ. To dive deeper into the mechanism of how S100A9 inhibition impairs CLL progression, we performed a Nanostring PanCancer immune profiling. Our data demonstrated significant downregulation in transcription factors (Fos, Jun and Egr1) downstream to the MAPK pathway in PaQ treated CD19+ cells compared to control. Corresponding to our flow cytometry data, PD-L1 and IL-10 gene expression were also decreased.
CONCLUSION: S100A9 inhibition not only impairs CLL growth in vivo, it also decreased the CLL-induced immune dysfunction, probably through a MAPK pathway dependent mechanism. The data demonstrates for the first time the significance of S100A9 targeting in CLL as a novel immunotherapeutic approach.
Disclosures
Pinilla Ibarz:SecuraBio: Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy; Pharmacyclics: Consultancy; Janssen Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal